Abstract
Introduction: Treatment results in diffuse large B-cell lymphoma (DLBCL) are heterogeneous. Established risk models like the International Prognostic Index (IPI) and molecular lymphoma features such as MYC translocations and the cell of origin (COO) subtype are prognostic of outcome. A positive iPET scan after 2 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) has recently been shown to predict poor outcome independent of the IPI (Positron Emission Tomography-Guided Therapy of Aggressive Non-Hodgkin Lymphomas trial [PETAL]; Dührsen et al., J Clin Oncol 36:2024, 2018). Another PET-derived parameter of potential prognostic significance is baseline MTV. This retrospective analysis of lymphoma biopsies from the PETAL trial investigated the relationship between molecular lymphoma features and PET parameters.
Methods: Available lymphoma specimens were analyzed for COO by immunohistochemistry employing the Hans-classifier (HC) and by gene expression (GE) using the HTG EdgeSeq System (HTG Molecular Diagnostics). MYC and BCL2 and/or BCL6 translocations ("double-hit" [DH]) were assessed by fluorescence in situ hybridization (FISH). MTV was determined applying the 41% SUVmax segmentation method, and iPET was evaluated using the deltaSUVmax method. Association between iPET result and molecular lymphoma features was assessed by risk ratios (RR). Survival curves of time-to-event endpoints were compared using hazard ratios (HR) from Cox regression and the log-rank test.
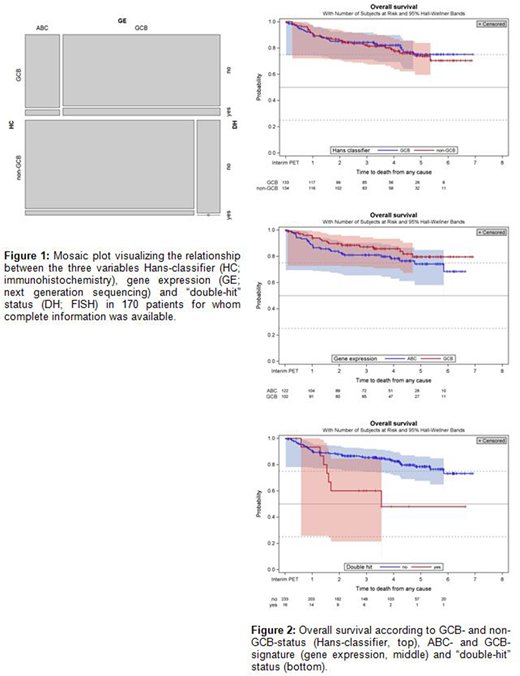
Results: Of 609 DLBCL patients treated in the PETAL trial, 63 had a positive iPET. 134 of 267 DLBCL biopsies available for HC analysis (50.2%) were classified as non-germinal center B-cell (non-GCB) and 133 (49.8%) as GCB. COO analysis by GE revealed an activated B-cell (ABC) type in 122 (51.1%) and a GCB type in 102 (42.7%) of 239 available biopsies (n=7 [2.9%] unclassified, n=8 [3.3%] failed quality control). Concordance between HC and GE was found in 165 of 197 biopsies studied by both methods (83.8%). MYC breaks were found in 27 (10.7%) and MYC amplifications in 48 (19.0%) of 253 cases studied by FISH. A DH lymphoma was diagnosed in 16 of 253 cases (6.3%). Complete information on HC, GE and DH status was available for 170 cases. The relationship is depicted in figure 1. COO classification by either HC or GE was not correlated with baseline MTV, iPET result, event-free (EFS) survival or overall (OS) survival. By contrast, DH status was correlated with positive iPET (RR 2.30 [95% CI, 0.76 to 6.96]) and inferior outcome as shown in figure 2 (EFS: HR 2.04 [95% CI, 1.02 to 4.07]; p=.044; OS: HR 3.00 [95% CI, 1.34 to 6.71]; p=.007). There was no correlation between DH status and MTV. iPET-positive DLBCL harbored MYC breaks more frequently than iPET-negative DLBCL (RR 3.29 [95% CI, 1.40 to 7.77]). A similar trend was observed in 72 cases tested for BCL2 breaks (RR 1.30 [95% CI, 0.44 to 3.84]) and 74 cases tested for BCL6 breaks (RR 1.85 [95% CI, 0.59 to 5.80]).
Conclusion: HC and GE showed good concordance with respect to COO classification, but COO was not correlated with MTV, iPET, EFS or OS. By contrast, MYC-rearranged lymphomas with or without BCL2 or BCL6 breaks were statistically significantly associated with a positive iPET, and DH lymphomas were correlated with poor outcome. Yet, the unfavorable prognosis of iPET-positive DLBCL cannot solely be explained by MYC translocations because most iPET-positive lymphomas lacked this genetic anomaly. Our results strengthen the role of iPET as a prognostic tool, independent not only of IPI, but also of COO status and MYC translocation.
Richter:HTG Molecular Diagnostics, Inc.: Research Funding. Hüttmann:Celgene: Other: Travel expenses; Roche: Other: Travel expenses. Gärtner:HTG Molecular Diagnostics, Inc.: Employment. Duehrsen:Amgen: Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Honoraria. Klapper:HTG Molecular Diagnostics, Inc.: Research Funding; Amgen: Honoraria, Research Funding; Regeneron: Honoraria, Research Funding; F.Hoffman-La Roche: Honoraria, Research Funding; Takeda: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.